KBMED Expands European Capacity with New Safety Pen-Type Blood Collection Needle Line
- KL KBMED
- Oct 13, 2025
- 2 min read
Irvine, California — KB Medical Group (KBMED), a global leader in medical device innovation and manufacturing, has announced the expansion of its production capacity with a new Safety Pen-Type Blood Collection Needle (Model: SBCN-3) line, scheduled to begin mass production in November 2025. |
This milestone marks a major investment in enhancing supply stability for Europe’s laboratory and hospital sectors, where the demand for safety-engineered blood collection devices continues to rise amid stricter occupational safety standards and growing healthcare needs. |
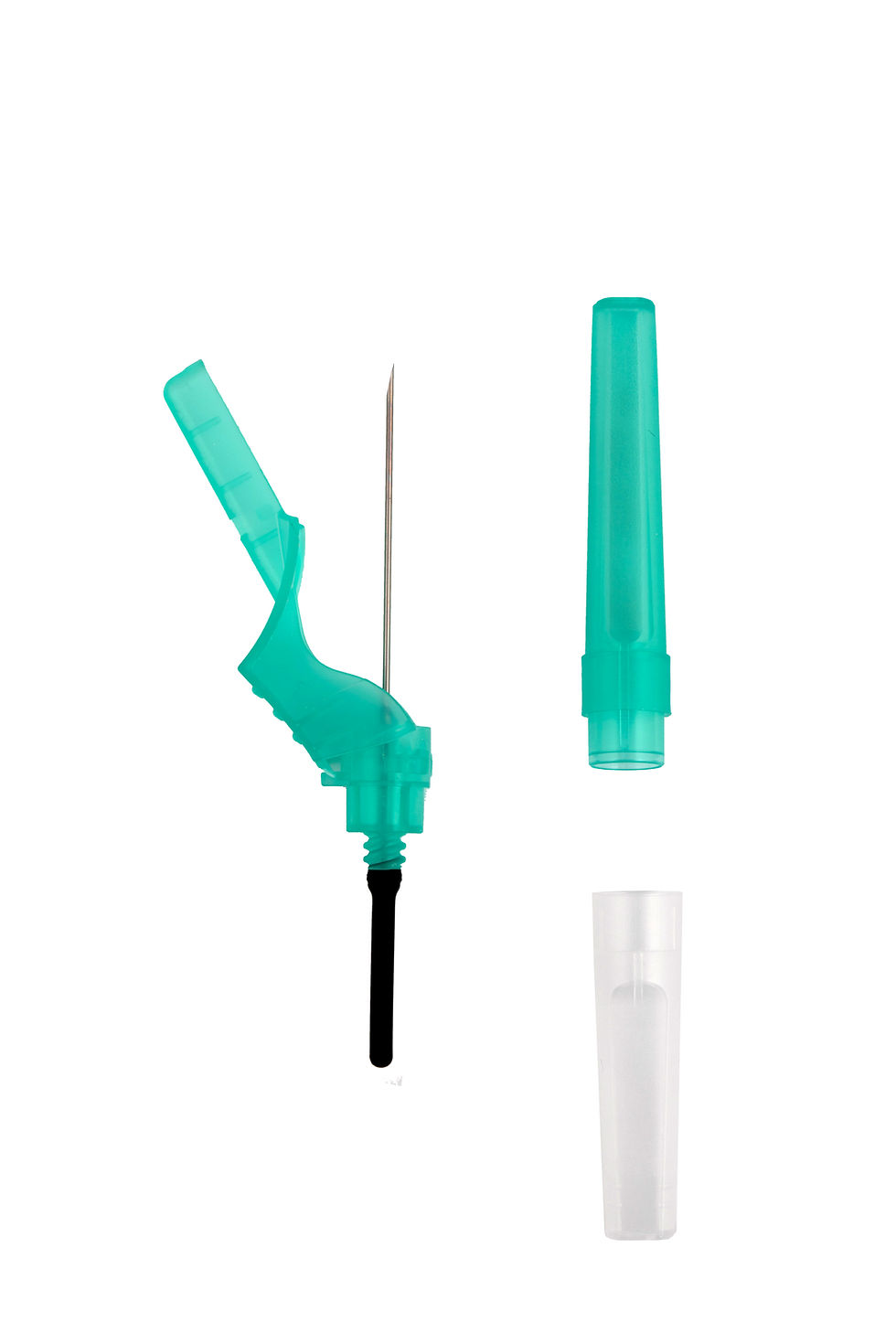
Designed for Safety and EfficiencyThe new SBCN-3 Safety Pen-Type Blood Collection Needle is designed to minimize the risk of needlestick injuries, protecting healthcare workers while maintaining comfort and precision during venipuncture.Its advanced safety design includes:
🔗 Explore Product Details |
Strengthening the European Supply Chain
With this new production line, KBMED reinforces its commitment to the European market, addressing ongoing supply chain shortages in safety blood collection devices. The automated facility integrates advanced process control, high-precision molding, and real-time quality monitoring, ensuring consistent, large-scale output with international compliance.
“Europe remains one of our most strategic markets,” said Ruffy L, Sales Manager at KBMED. “The addition of this production line reflects our commitment to supporting laboratory and hospital partners with reliable, cost-effective, and safe solutions that meet the highest standards.”
Synergized Capacity for Tubes and Needles
In parallel, KBMED’s vacuum blood collection tube production has reached a capacity exceeding 100 million units per month, creating strong synergy between its tube and needle lines. This integrated manufacturing capability enables KBMED to provide a complete and scalable venous blood collection solution, ensuring supply reliability and compatibility across all clinical applications.
“This expansion demonstrates our long-term commitment to Europe and our vision to lead with quality, safety, and service,” said Susan S, CEO of KBMED. “Our mission is to make medical safety accessible and dependable worldwide.”

This achievement underscores KBMED’s continued efforts to enhance service experience and deepen supply chain collaboration worldwide. It also represents another step forward in the company’s strategic expansion across the Americas and global markets.Looking ahead, KBMED remains committed to its philosophy of “Quality Without Borders, Service Without Limits.” By combining innovation, precision manufacturing, and efficient logistics, KBMED continues to deliver world-class medical products and solutions that exceed customer expectations. |









